DIMETHYL SUCCINATE
PRODUCT IDENTIFICATION
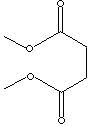
146.14
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
1.115 - 1.119
SOLUBILITY IN WATER
NFPA RATINGS
REFRACTIVE INDEX
85 C
APPLICATIONS
Succinic Acid (Butanedioic Acid) is a dicarboxylic acid of four carbon atoms. It occurs naturally in plant and animal tissues. It plays a significant role in intermediary metabolism (Krebs cycle) in the body. Krebs cycle (also called citric acid cycle tricarboxylic acid cycle) is a sequence process of enzymatic reaction which a two-carbon acetyl unit is oxidized to carbon dioxide and water to provide energy in the form of high-energy phosphate bonds. Succinic acid is a colourless crystalline solid with a melting point of 185 -187 C soluble in water slightly dissolved in ethanol, ether, acetone and glycerine not dissolved in benzene, carbon sulfide, carbon tetrachloride and oil ether. The common method of synthesis of succinic acid is the catalytic hydrogenation of maleic acid or its anhydride. Carboxylic acid can yield acyl halides, anhydrides, esters, amides, and nitriles for the application of drug, agriculture, and food products, and other industrial uses. Dimethyl Succinate is used in preparing pharmaceuticals, agrochemicals and perfumery products. It is used in manufacturing additives, plastics and other organic compounds.
APPEARANCE
ESTERS CONTENT
98.5% min
ACIDITY
0.2 mg KOH/g max
MOISTURE
0.1% max
REFRACTIVE INDEX
1.41 - 1.43 at 20 C
COLOR (HAZEN)
20 max
OTHER INFORMATION
- Plasticizer for polymers
- Biodegradable solvents and lubricants
- Engineering plastics
- Epoxy curing agent
- Adhesive and powder coating
- Corrosion inhibitor
- Perfumery and pharmaceutical
- Electrolyte
There are almost infinite esters obtained from carboxylic acids. Esters are formed by removal of water from an acid and an alcohol. Carboxylic acid esters are used as in a variety of direct and indirect applications. Lower chain esters are used as flavouring base materials, plasticizers, solvent carriers and coupling agents. Higher chain compounds are used as components in metalworking fluids, surfactants, lubricants, detergents, oiling agents, emulsifiers, wetting agents textile treatments and emollients, They are also used as intermediates for the manufacture of a variety of target compounds. The almost infinite esters provide a wide range of viscosity, specific gravity, vapor pressure, boiling point, and other physical and chemical properties for the proper application selections.
|
Structure & Length |
Common Name |
Formula |
Melting Point |
|
Straight C2 |
Oxalic Acid (Ethanedioic Acid) |
HOOCCOOH |
187 C |
|
Straight C3 |
Malonic Acid (Propanedioic Acid) |
HOOCCH2COOH |
136 C |
|
Straight C4 |
Succinic Acid (Butanedioic Acid) |
HOOC(CH2)2COOH |
190 C |
|
Straight C5 |
Glutaric Acid (Pentanedioic Acid) |
HOOC(CH2)3COOH |
99 C |
|
Straight C6 |
Adipic Acid (Hexanedioic Acid) |
HOOC(CH2)4COOH |
152 C |
|
Straight C7 |
Pimelic Acid (Heptanedioic Acid) |
HOOC(CH2)5COOH |
106 C |
|
Straight C8 |
Suberic Acid (Octanedioic Acid) |
HOOC(CH2)6COOH |
143 C |
|
Straight C9 |
Azelaic Acid (Nonanedioic Acid) |
HOOC(CH2)7COOH |
106 C |
|
Straight C10 |
Sebacic Acid (Decanedioic Acid) |
HOOC(CH2)8COOH |
134 C |
There are almost infinite esters obtained from thousands of potential starting materials. Esters are formed by removal of water from an acid and an alcohol, e.g., carboxylic acid esters, phosphoric acid esters, and sulfonic acid esters. Carboxylic acid esters are used as in a variety of direct and indirect applications. Lower chain esters are used as flavouring base materials, plasticizers, solvent carriers and coupling agents. Higher chain compounds are used as components in metalworking fluids, surfactants, lubricants, detergents, oiling agents, emulsifiers, wetting agents textile treatments and emollients, They are also used as intermediates for the manufacture of a variety of target compounds. The almost infinite esters provide a wide range of viscosity, specific gravity, vapor pressure, boiling point, and other physical and chemical properties for the proper application selections.